CBSE Notes Class 10 Science (Chemistry) Chapter 3 – Metals and Non- metals
Corrosion
Alloys
Alloys are homogeneous mixtures of metal with other metals or
nonmetals. Alloy formation enhances the desirable properties of
the material, such as hardness, tensile strength and resistance to
corrosion.
Examples of a few alloys –
Brass: copper and zinc
Bronze: copper and tin
Solder: lead and tin
Amalgam: mercury and other metal
Corrosion
Gradual deterioration of material usually a metal by the action of moisture, air or chemicals in the surrounding environment.
Rusting:
4Fe(s)+3O2(from air)+xH2O(moisture)→2Fe2O3. xH2O(rust)
Corrosion of copper:
Cu(s)+H2O(moisture)+CO2(from air)→CuCO3.Cu(OH)2(green)
Corrosion of silver:
Ag(s)+H2S(from air)→Ag2S(black)+H2(g)
Prevention of Corrosion
Prevention:
1. Coating with paints or oil or grease: Application of paint or oil or grease on metal surfaces keep out air and moisture.
2. Alloying: Alloyed metal is more resistant to corrosion. Example: stainless steel.
3. Galvanization: This is a process of coating molten zinc on iron articles. Zinc forms a protective layer and prevents corrosion.
4. Electroplating: It is a method of coating one metal with another
by the use of electric current. This method not only lends protection
but also enhances the metallic appearance.
Example: silver plating, nickel plating.
5. Sacrificial protection: Magnesium is more reactive than iron. When it is coated on the articles made of iron or steel, it acts as the cathode, undergoes reaction (sacrifice) instead of iron and protects the articles.
Physical Properties
Physical Properties of Metals
● Hard and have a high tensile strength
● Solid at room temperature
● Sonorous
● Good conductors of heat and electricity
● Malleable, i.e., can be beaten into thin sheets
● Ductile, i.e., can be drawn into thin wires
● High melting and boiling points (except Caesium (Cs) and Gallium (Ga))
● Dense, (except alkali metals). Osmium – highest density and lithium – least density
● Lustrous
● Silver-grey in colour, (except gold and copper)
Non-Metals
Nonmetals are those elements which do not exhibit the properties of metals.
Physical Properties of Nonmetals
- Occur as solids, liquids and gases at room temperature
- Brittle
- Non-malleable
- Non-ductile
- Non-sonorous
- Bad conductors of heat and electricity
Exceptions in Physical Properties
- Alkali metals (Na, K, Li) can be cut using a knife.
- Mercury is a liquid metal.
- Lead and mercury are poor conductors of heat.
- Mercury expands significantly for the slightest change in temperature.
- Gallium and caesium have a very low melting point.
- Iodine is non-metal but it has lustre.
- Graphite conducts electricity.
- Diamond conducts heat and has a very high melting point.
Chemical Properties
Chemical Properties of Metals
● Alkali metals (Li, Na, K, etc) react vigorously with water and oxygen or air.
● Mg reacts with hot water.
● Al, Fe and Zn react with steam.
● Cu, Ag, Pt, Au do not react with water or dilute acids.
Reaction of Metals with Oxygen (Burnt in Air)
Metal + Oxygen→ Metal oxide (basic)
● Na and K are kept immersed in kerosene oil as they react vigorously with air and catch fire.
4K(s)+O2(g)→2K2O(s) (vigorous reaction)
● Mg, Al, Zn, Pb react slowly with air and form a protective layer that prevents corrosion.
2Mg(s)+O2(g)→2MgO(s) (Mg burns with white dazzling light)
4Al(s)+3O2(g)→2Al2O3(s)
● Silver, platinum and gold don’t burn or react with air.
Basic Oxides of Metals
Some metallic oxides get dissolved in water and form alkalis. Their aqueous solution turns red litmus blue.
Na2O(s)+H2O(l)→2NaOH(aq)
K2O(s)+H2O(l)→2KOH(aq)
Amphoteric Oxides of Metals
Amphoteric oxides are metal oxides which react with both acids as well as bases to form salt and water.
For example – Al2O3,ZnO,PbO,SnO
Al2O3(s)+6HCl(aq)→2AlCl3(aq)+3H2O(l)
Al2O3(s)+2NaOH(aq)→2NaAlO2(aq)+H2O(l)
ZnO(s)+2HCl(aq)→ZnCl2(aq)+H2O(l)
ZnO(s)+2NaOH(aq)→Na2ZnO2(aq)+H2O(l)
Reactivity Series
The below table illustrates the reactivity of metals from high order to low order.
| Symbol | Element |
| K | Potassium ( Highly Active Metal) |
| Ba | Barium |
| Ca | Calcium |
| Na | Sodium |
| Mg | Magnesium |
| Al | Aluminium |
| Zn | Zinc |
| Fe | Iron |
| Ni | Nickel |
| Sn | Tin |
| Pb | Lead |
| H | Hydrogen |
| Cu | Copper |
| Hg | Mercury |
| Ag | Silver |
| Au | Gold |
| Pt | Platinum |
Reaction of Metals with Water or Steam
Metal+Water→Metalhydroxide or Metaloxide+Hydrogen
2Na+2H2O(cold)→2NaOH+H2+heat
Ca+2H2O(cold)→Ca(OH)2+H2
Mg+2H2O(hot)→Mg(OH)2+H2
2Al+3H2O(steam)→Al2O3+3H2
Zn+H2O(steam)→ZnO+H2
3Fe+4H2O(steam)→Fe3O4+4H2
Reaction of Metals with Acid
Metal+diluteacid→Salt+Hydrogengas
2Na(s)+2HCl(dilute)→2NaCl(aq)+H2(g)
2K(s)+H2SO4(dilute)→K2SO4(aq)+H2(g)
Only Mg and Mn, react with very dilute nitric acid to liberate hydrogen gas.
Mg(s)+2HNO3(dilute)→Mg(NO3)2(aq)+H2(g)
Mn(s)+2HNO3(dilute)→Mn(NO3)2(aq)+H2(g)
Displacement Reaction
A more reactive element displaces a less reactive element from its compound or solution.
How Do Metals React with Solution of Other Metal Salts
Metal A+Salt of metal B → Salt of metal A + Metal B
Fe(s)+CuSO4(aq)→FeSO4(aq)+Cu(s)
Cu(s)+2AgNO3(aq)→Cu(NO3)(aq)+2Ag(s)
Reaction of Metals with Bases
Base+metal → salt+hydrogen
2NaOH(aq)+Zn(s) → Na2ZnO2(aq)+H2(g)
2NaOH(aq)+2Al(s)+2H2O(l) → 2NaAlO2(aq)+2H2(g)
Extraction of Metals and Non-Metals
Applications of Displacement Reaction
Uses of displacement reaction
- Extraction of metals
- Manufacturing of steel
- Thermite reaction: Al(s)+Fe2O3(s) → Al2O3+Fe(molten)
The thermite reaction is used in welding of railway tracks, cracked machine parts, etc.
Occurrence of Metals
Most of the elements, especially metals occur in nature in the
combined state with other elements. All these compounds of metals are
known as minerals. But out of them, only a few are viable sources of that metal. Such sources are called ores.
Au, Pt – exist in the native or free state.
Extraction of Metals
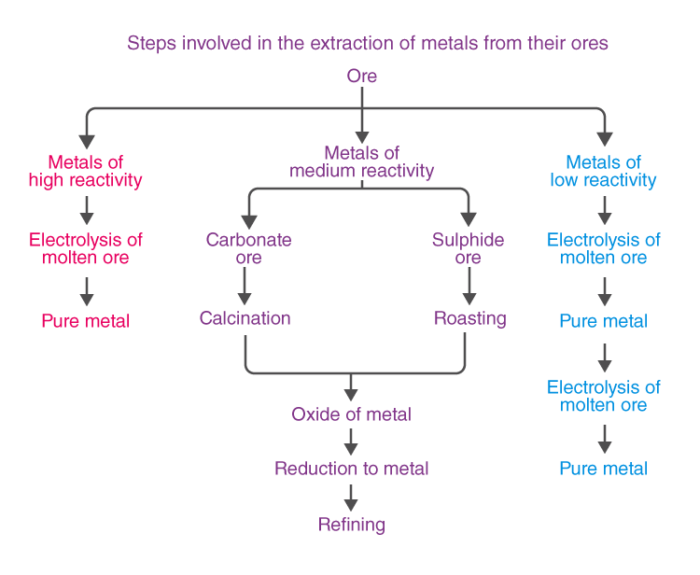
Metals of high reactivity – Na, K, Mg, Al.
Metals of medium reactivity – Fe, Zn, Pb, Sn.
Metals of low reactivity – Cu, Ag, Hg
Roasting
Converts sulphide ores into oxides on heating strongly in the presence of excess air.
It also removes volatile impurities.
2ZnS(s)+3O2(g)+Heat→2ZnO(s)+2SO2(g)
Calcination
Converts carbonate and hydrated ores into oxides on heating strongly in the presence of limited air. It also removes volatile impurities.
ZnCO3(s)+heat→ZnO(s)+CO2(g)
CaCO3(s)+heat→CaO(s)+CO2(g)
Al2O3.2H2O(s)+heat→2Al2O3(s)+2H2O(l)
2Fe2O3.3H2O(s)+heat→2Fe2O3(s)+3H2O(l)
Extracting Metals Low in Reactivity Series
By self-reduction- when the sulphide ores of less electropositive metals like Hg, Pb, Cu etc., are heated in air, a part of the ore gets converted to oxide which then reacts with the remaining sulphide ore to give the crude metal and sulphur dioxide. In this process, no external reducing agent is used.
1. 2HgS(Cinnabar)+3O2(g)+heat→2HgO(crude metal)+2SO2(g)
2HgO(s)+heat→2Hg(l)+O2(g)
2. Cu2S(Copperpyrite)+3O2(g)+heat→2Cu2O(s)+2SO2(g)
2Cu2O(s)+Cu2S(s)+heat→6Cu(crude metal)+SO2(g)
3. 2PbS(Galena)+3O2(g)+heat→2PbO(s)+2SO2(g)
PbS(s)+2PbO(s)→2Pb(crudemetal)+SO2(g)
Extracting Metals in the Middle of Reactivity Series
Smelting – it involves heating the roasted or calcined ore (metal
oxide) to a high temperature with a suitable reducing agent. The crude
metal is obtained in its molten state.
Fe2O3+3C(coke)→2Fe+3CO2
Aluminothermic reaction – also known as the Goldschmidt reaction is a
highly exothermic reaction in which metal oxides usually of Fe and Cr
are heated to a high temperature with aluminium.
Fe2O3+2Al→Al2O3+2Fe+heat
Cr2O3+2Al→Al2O3+2Cr+heat
Extraction of Metals Towards the Top of the Reactivity Series
Electrolytic reduction:
1. Down’s process: Molten NaCl is electrolysed in a special apparatus.
At the cathode (reduction):
Na+(molten)+e−→Na(s)
Metal is deposited.
At the anode (oxidation):
2Cl−(molten)→Cl2(g)+2e–
Chlorine gas is liberated.
2. Hall’s process: Mixture of molten alumina and a fluoride solvent usually cryolite, (Na3AlF6) is electrolysed.
At the cathode (reduction):
2Al3++6e–→ 2Al(s)
Metal is deposited.
At the anode (oxidation):
6O2– → 3O2(g)+12e–
Oxygen gas is liberated.
Enrichment of Ores
It means the removal of impurities or gangue from ore, through various physical and chemical processes. The technique used for a particular ore depends on the difference in the properties of the ore and the gangue.
Refining of Metals
Refining of metals – removing impurities or gangue from crude metal. It is the last step in metallurgy and is based on the difference between the properties of metal and the gangue.
Electrolytic Refining
Metals like copper, zinc, nickel, silver, tin, gold etc., are refined electrolytically.
Anode: impure or crude metal
Cathode: a thin strip of pure metal
Electrolyte: aqueous solution of metal salt
From anode (oxidation): metal ions are released into the solution
At cathode (reduction): the equivalent amount of metal from solution is deposited
Impurities deposit at the bottom of the anode.
The Why Questions
Electronic Configuration
Group 1 elements – Alkali metals
| Element | Electronic Configuration |
| Lithium(Li) | 2,1 |
| Sodium(Na) | 2,8,1 |
| Potassium(K) | 2,8,8,1 |
| Rubidium(Rb) | 2,8,18,8,1 |
Group 2 elements – Alkaline earth metals
| Element | Electronic Configuration |
| Beryllium(Be) | 2,2 |
| Magnesium(Mg) | 2,8,2 |
| Calcium(Ca) | 2,8,8,2 |
| Stronium(Sr) | 2,8,18,8,2 |
How Do Metals and Nonmetals React
Metals lose valence electron(s) and form cations.
Non-metals gain those electrons in their valence shell and form anions.
The cation and the anion are attracted to each other by strong electrostatic force, thus forming an ionic bond.
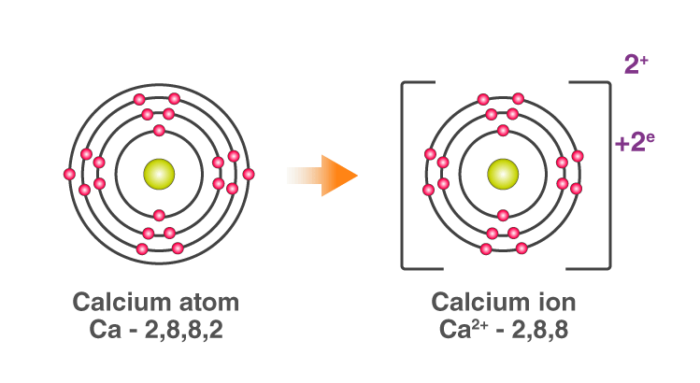
For example: In calcium chloride, the ionic bond is formed by opposite charged calcium and chloride ions.
Calcium atom loses 2 electrons and attains the electronic configuration
of the nearest noble gas (Ar). By doing so, it gains a net charge of +2.
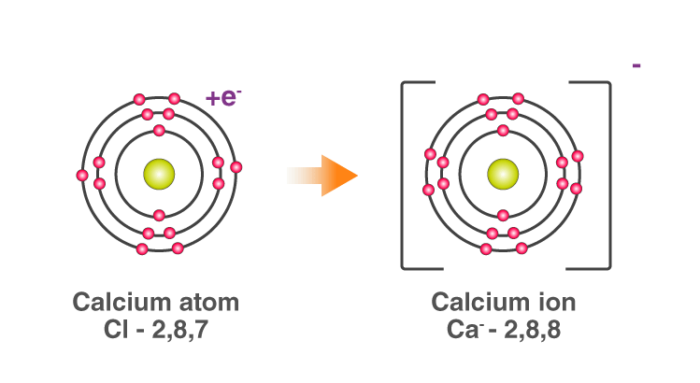
The two Chlorine atoms take one electron each, thus gaining a charge of -1 (each) and attain the electronic configuration of the nearest noble gas (Ar).
Ionic Compounds
The electrostatic attractions between the opposite charged ions hold the compound together.
Example: MgCl2, CaO, MgO, NaCl etc.
Properties of Ionic Compound
Ionic compounds
- Are usually crystalline solids (made of ions).
- Have high melting and boiling points.
- Conduct electricity when in aqueous solution and when melted.
- Are mostly soluble in water and polar solvents.
Physical Nature
Ionic solids usually exist in regular, well-defined crystal structures.
Electric Conduction of Ionic Compounds
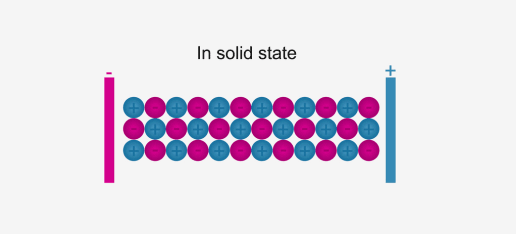
Ionic compounds conduct electricity in the molten or aqueous state when ions become free and act as charge carriers.
In solid form, ions are strongly held by electrostatic forces of
attractions and are not free to move; hence do not conduct electricity.
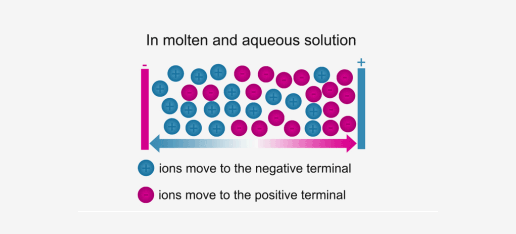
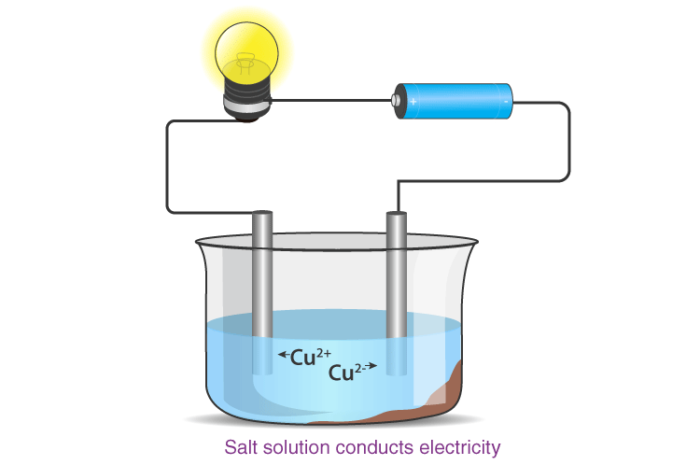
Melting and Boiling Points of Ionic Compounds
In ionic compounds, the strong electrostatic forces between ions require a high amount of energy to break. Thus, the melting point and boiling point of an ionic compound are usually very high.
Solubility of Ionic Compounds
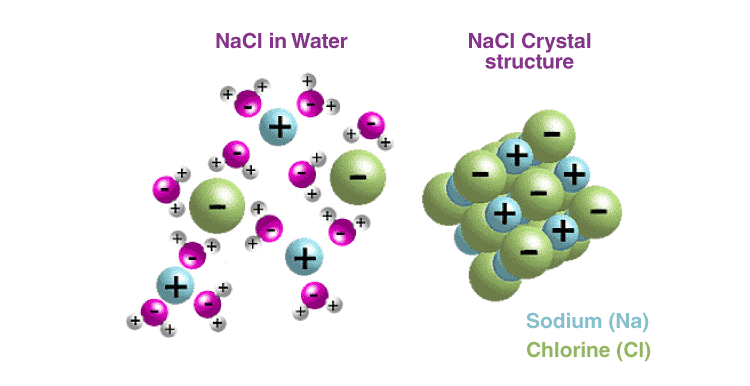
Most ionic compounds are soluble in water due to the separation of ions by water. This occurs due to the polar nature of water.
For example, NaCl is a 3-D salt crystal composed of Na+ and Cl−
ions bound together through electrostatic forces of attractions. When a
crystal of NaCl comes into contact with water, the partial positively
charged ends of water molecules interact with the Cl− ions, while the negatively charged end of the water molecules interacts with the Na+
ions. This ion-dipole interaction between ions and water molecules
assist in the breaking of the strong electrostatic forces of attractions
within the crystal and ultimately in the solubility of the crystal.
Vikash Kumar
left-sidebar
full-width
right-sidebar




Thankyou
ReplyDeleteFor more update also subscribe my YouTube Channel
ReplyDeleteUnique Education Portal
If you have doubt, comment me.
Thank you for visiting.